1791 – 1867

| NAME | DATE |
On a hovercraft, electricity is needed to operate navigation lights, to start the engine, to ignite the fuel, to operate a radio, to use the horn, to charge the onboard battery, and sometimes to run an onboard computer. Hovercraft can also generate a huge static electricity buildup when the skirt slides across dry ground. One key figure in the study of electrical phenomena was Michael Faraday.
Michael Faraday
1791 – 1867

Michael Faraday, considered by some to be the greatest experimentalist in history, was an English bookbinder who developed an interest in chemistry and physics from the works on the subject that passed through the shop where he worked. He taught himself the basic principles of science, and soon was assistant to the chemist Sir Humphry Davy in the laboratory of the Royal Institution in London. There, he studied a variety of subjects in experimental chemistry until, inspired by Davy’s experiments in electrolysis, he joined in the embryonic study of electromagnetism. When, in 1820, Hans Oersted and Andre Marie Ampere demonstrated that an electric current produced a magnetic field, Faraday applied his electromagnetic theory to that discovery and postulated that the reverse should also be true: that a magnetic field would produce an electric current. Although his overall theory, a grand scheme in which heat, light, magnetism, and chemical forces were all different aspects of some unified natural force, turned out to be wrong, in 1831 he demonstrated electromagnetic induction. This was a discovery that had great implications for the world at large, since it made possible the dynamo, which formed the basis for the widespread generation of electricity for mass consumption as well as for the electrical generation required for internal combustion engines, such as those in hovercraft.
Faraday’s Law:
V = −N × Δ(B × A) / Δt
Voltage generated = −(Number of turns in the coil) × Change in (Magnetic field
strength × Area of the coil) / Amount of time
Electromagnetic induction means that any time you have an electrically conductive loop and a magnetic field, a change in the magnetic field flowing through the conductor causes a current to flow. Magnetic flux is the section of the conductor through which the magnetic field is flowing. The mechanism does not matter: it is irrelevant whether the magnet is flying through the loop, the loop is rolling through the field, or the magnet is getting stronger or weaker. As long as you have some change for some reason in the magnetic field B, the coil area within the field A, or both, you get current (although you could have the two change so that their changes cancel each other). The faster the change, the higher the voltage you get. This principle is used in hovercraft to generate electricity to run the engines.
Another interesting electrical phenomenon is often referred to as static electricity. To understand what static electricity really means, we need to look at the atomic level of objects with which we interact daily. Atoms are composed of many different particles, the most prominent of which are protons, neutrons, and electrons. The force that controls the interactions between these subatomic particles is the electromagnetic force, also known as the Coulomb force, named after the 18th century French physicist Charles Augustin Coulomb.
Charles Augustin Coulomb
1736 – 1806

Coulomb was trained as an engineer in the army, which brought him to the island of Martinique to engineer the construction of a new fort. The fort was completed, but the illnesses he experienced during his three-year stay permanently damaged his health. Upon his return to France, he began to produce and present scientific works dealing with applied mechanics. In 1781, he published a work on static and fluid friction that earned Coulomb a permanent post in the Académie des Sciences. Thereafter, he removed himself from engineering, except in a consulting role, and devoted himself to science. It was during this period that he wrote seven treatises on electricity and magnetism, including the formulation of the physical law that bears his name and describes the electromagnetic force. During the French Revolution, the Académie des Sciences ceased to exist and Coulomb retired to a country house to continue his research. After the Revolution, he returned to Paris, married the mother of his two children, and took a position at the Institut de France. He worked until 1806 as the Inspector General of Public Instruction; during these years, Coulomb establised lycées (public schools for older students) across France.
Coulomb’s Law describes the electromagnetic force between two objects as depending on the charges on the objects and inversely on the square of the distance between them.
F = k × q1 ×
q2/r2
Force = k × Charge on one object × Charge on other object /
(Distance between objects)2
In this equation, k is a constant equal to 1 / (4 × π × ε0) and ε0 is a fundamental physical constant known as the permittivity of free space. The value of k, also known as the electrostatic constant or the Coulomb force constant is approximately 8.99×109 N-m2/C2 [3.13×1012 lb-in2/C2].
Example 1: Solution: F = k × q1
× q2/r2 As you can see, the form of the equation would not change significantly with the different particles involved. The signs of the charges would change, so the signs of the solutions would change, but the magnitude of the force would stay the same. Therefore, rather than performing the entire calculation two additional times, we can instead consider whether the signs are positive or negative. The charges of the two protons are both positive, so the product is also positive. Since it then becomes a product of three positive numbers, the force is positive. The charges of two electrons are both negative, so the product is positive and the force, again, is positive. Now, think about what it means for the force to be positive or negative. Remember that force is a vector quantity, so we must consider the direction as well as the magnitude of the force. Since the forces are the same in everything but the sign, we know that whatever the direction is, each pulls or pushes in the exact opposite direction from the other. However, what is that direction? Think about what the force does: it causes the two particles to move toward or away from each other; that is, it regulates the distance between them. It makes sense, therefore, that the force should be directly related to the distance between the particles. If the force is negative, it tries to make the distance smaller, making it an attractive force. Similarly, a positive force tries to make the distance larger, making it a repulsive force. This is why opposites attract and likes repel. We have determined that two particles 2 ×10−7 m [7.87×10−7 in] apart with charge magnitude of e will influence each other with a force of −5.767×10−15 N [−1.297×10−15 lb]. If the particles are opposite in charge then they will attract. |
What about the effect of gravity? Gravity must be a pretty potent force for it to hold everything on earth and to keep the planets revolving around the sun. After all, gravity is generally considered to be the force that shaped the universe as we know it. Let us now determine the force of gravity between these same sets of particles.
Gravitational force is determined by a formula similar to that used to determine the Coulomb force, except that instead of k it uses a constant G, equal to 6.67 ×10−11 N-m2/kg2 [4.954×10−6 lb-in2/slug2]. Also, the force of gravity depends on the masses of the objects rather than on the charges, and it is always attractive. The equation for the magnitude of the gravitational force between two bodies is Fgravity =G × m1 × m2 / r2. The mass of a proton is 1.6726 ×10−27 kg [0.1145×10−27 slug] and the mass of an electron is 9.1095×10−31 kg [0.624×10−31 slug]. Thus, the force between two protons will be the largest and, at the same distance as the previous example, that force will be 4.665×10−51 N [1.004×10−51 lb]. Scientists believe this system describes the way the universe works. There are four fundamental forces: gravity, electromagnetism, the strong nuclear force, and the weak nuclear force. Gravity is weaker than the second weakest (known as the Weak Force) by a factor of 1.67×1033. The reason that gravity seems to be the biggest player in existence is that two of the forces cannot reach beyond atomic nuclei and the fourth, electromagnetism, requires objects to be electrically charged. Most objects have the same number of electrons as they do protons, so the charges cancel each other, and the electromagnetic force does not apply to them. On the other hand, stars, the planets that revolve around them, and the matter on the planets (rocks, people, clouds, etc.) all have considerable mass, so the gravitational force tends to dominate at that level.
Sometimes it is possible to transfer charged particles, usually electrons, from one object to another. This often results from contact between two surfaces due to the triboelectric effect, which occurs when two materials come into contact in certain combinations. The two materials in contact form a slight chemical bond by sharing electrons, and when they are separated again it is possible for one to come away with some of the electrons from the other material. Materials Scientists have developed a list, the triboelectric series, in which materials at one end (such as fur, hair, dry skin, lead, and glass) tend to give up electrons and gain a positive charge in these situations. In the middle of the list, materials such as cotton and steel do not have tendencies to go either way. Most types of rubbers and plastics, and a number of metals, appear on the end of the list with materials that tend to gain extra electrons, and thus negative charge, in these situations. This phenomenon is used in Van de Graff generators, which employ two rollers that are far apart on the triboelectric series, with a belt running between them. As a point on the belt passes the lower roller, it picks up some electrons. It then deposits the electrons on the other roller, which then transfers them through an electrode to a metal sphere. The sphere, being a hollow conductor, acts as a Faraday Cage, which means that the electrons distribute themselves evenly around the outside of the sphere. When someone touches the sphere, some of the extra electrons move, giving the person a net negative charge. When the person’s hair becomes negatively charged, the Coulomb Force causes individual hairs to try to move as far as possible from each other, resulting in the hair standing out in all directions.

Figure 29-1. Negatively charged hairs
Image courtesy Fundamentals of Physics; D. Halliday, R. Resnik
and J. Walker
This phenomenon also occurs in hovercraft. Contact between the skirt and the ground can result in electrons moving to or away from the skirt, and the rest of the hovercraft. In addition, particles of dust or snow moving with the hovercraft lift and thrust air impact the hovercraft’s plastic or painted duct and generate positive or negative charges. This process is similar to the operation of a Van de Graff generator. The static electricity thus produced can interfere with electronic components, adding an extra challenge to designing such things for hovercraft.
When charges are put into motion, electrical current is the result. Wearing only socks, scrape your feet across a carpet. This will rub electrons off the carpet and onto you, giving your body a charge. If you then put your finger close to a metal door knob, you’ll see a spark and feel a jolt as the excess electrons on your body jump onto the doorknob. This is the same electricity supplied by batteries and wall sockets. Electrons flowing through power wires provide the electricity that powers everything in your hovercraft. All electrical current consists of charges in motion.
How do electrons move through a wire to create electricity? To answer this question, consider the atomic structure of the metal in the wire. Metals, such as copper, silver, gold, aluminum, and brass are conductors. Conductors allow charges to easily move through them.
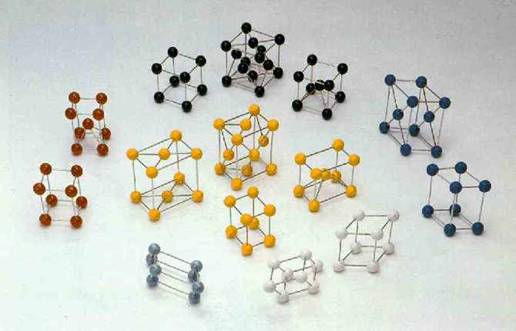
Figure 29-2. Atomic arrangement in different metals.
Image ©2005 DiscoverHover
Figure 29-2 shows how the atoms in conductors are typically arranged. Each large ball represents the nucleus of an atom. Notice all the space between the nuclei (plural of nucleus). The electrons of each atom are free to roam in this space, jumping from one atom to another. When extra electrons are placed on a conductor, they are free to move about. This is why electricity flows so easily through conductors.
Plastic, glass, rubber, and wood are all examples of insulators. Insulators do not allow electricity to pass through them. Unlike metals and other conductors, the atoms of insulators do not exchange electrons. When an atom of an insulator gets extra electrons, the electrons stay on that atom rather than spreading out across neighboring atoms. When you put a charge on an insulator, such as a hovercraft, the charge stays put and doesn’t spread across the material as it would on a conductor. Electrical wires always consist of copper or another conductor on the inside and plastic or another insulator on the outside. This funnels electricity through the inside of the wire, but keeps you from getting shocked when you touch the outside of the wire.
Quiz Questions:
| ©2005 World Hovercraft Organization All rights reserved. Copies of this Curriculum Guide may be printed for classroom use exclusively by DiscoverHover registered members. This Curriculum Guide and all materials contained in the DiscoverHover web site are protected by copyright laws and may not be reproduced, republished, distributed, or displayed on any other web site without the express prior written permission of the World Hovercraft Organization. |