| NAME |
|
Although we often think of gravity as a fairly strong force, on the scale of tiny things such as atoms and molecules, gravity becomes insignificant. Remember that the strength of the gravitational force depends on the mass of an object, so extremely light objects like atoms feel practically no gravitational force. Instead, other forces begin to play a large role in how atoms act. One is the electric force. While gravity is an attractive force based on the mass of objects, the electric force can be either attractive or repulsive, and is dependant on something called charge. There are two types of charges: positive and negative. When two charges are near each other, they create a force that can either try to pull them together or push them away, depending on the charges. In order to determine whether the force between the charges will be attractive or repulsive, remember the following rule:
Like charges repel each other, while unlike charges attract each other.
Two positive charges or two negative charges will repel each other,
but a positive and a negative charge attract one another.
Where exactly does charge come from? The answer to this lies in the microscopic
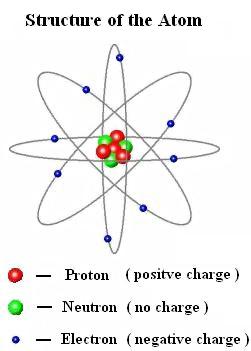
world of atoms. Atoms are made of three basic particles: protons, neutrons,
and electrons. Protons are tiny, positively charged
particles. Neutrons have about the same size and mass
as protons, but carry no charge. Together these particles are found in the nucleus
of an atom. This is the small but very dense core of every atom. Similar to
how the moon orbits Earth, electrons orbit around
the nucleus. Electrons are much smaller than protons and neutrons, and they
carry a negative charge. Since protons and electrons have opposite charges,
they attract each other. This force holds the electrons in orbit around the
nucleus, just like gravity holds the moon in orbit. The atom as a whole, however,
has no net charge because the negative charge of the electrons and the positive
charge of the protons cancel each other out.

Most atoms have the same amount of protons and electrons in order to keep a total charge of zero. Since electrons orbit on the outside of the atom, however, it is possible to remove some electrons and give the atom a positive charge, or add some electrons and give the atom a negative charge. Doing this to a large number of atoms in an object causes the object to become charged. Charged objects act in the same way that single charges do: opposite charges attract while like charges repel. When this charge remains on an object and doesn’t move, it is called static charge.
A Van de Graaff generator is a device that contains a large metal ball that gains a large negative charge. This is done by adding a lot of extra electrons to the ball. When someone touches the ball, some of the extra electrons pass over onto the person. This makes the person’s body (including the hair) negatively charged. Remember that like charges repel each other, so each negatively charged hair on the person’s body will repel each other. The hairs stick straight up, trying to separate as far as they can.

This condition can also occur in hovercraft. As the skirt of the
hovercraft drags along the ground, it can scrape electrons off of the grass
or ground. This can give the entire hovercraft a static charge. This makes designing
electronic equipment on the hovercraft difficult since it can be affected by
the static charge.
When charges are put into motion, electricity is the
result. Scrape your feet across a carpet wearing only socks. This will rub electrons
off the carpet and onto you, giving your body a charge. Put your finger close
to a metal door knob and you’ll see a spark and feel a jolt as the excess
electrons on you jump onto the doorknob. This is the same electricity that is
supplied by batteries and/or wall sockets. Electrons flowing through power wires
provide the electricity that powers everything in your home. All electricity
is simply charges in motion.
How do electrons move through a wire to create electricity? To solve this question,
look to the atomic structure of the metal in the wire. Metals, such as copper,
silver, aluminum, and brass are called conductors. Conductors
allow charges to easily move through them.
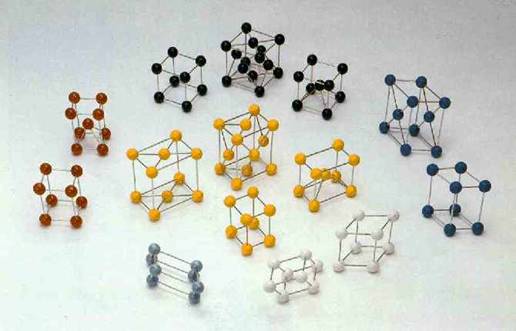
The diagram shows how the atoms in conductors are typically arranged.
Each large ball represents the nucleus of an atom. Notice all the space between
the nuclei (plural of nucleus). The electrons of each atom are free to roam
in this free space, jumping from one atom to another. When extra electrons are
placed on a conductor, they are free to move about. This is why electricity
flows so easily through conductors.
Plastic, glass, rubber, and wood are all examples of insulators. Insulators
do not allow electricity to pass through them. Unlike metals and other conductors,
the atoms of insulators do not exchange electrons. When an atom of an insulator
gets extra electrons put on it, the electrons stay on that atom rather than
spreading out across neighboring atoms. Because of this, when you put a charge
on an insulator, the charge stays put and doesn’t spread across the material
in the way charge does on conductors. Electrical wires always have copper or
some sort of conductor on the inside and plastic or another insulator on the
outside. This allows electricity to flow through the inside of the wire, but
keeps you from getting shocked when you touch the outside of the wire.